Sensitive, specific, precise, and accurate detection of low frequency mutations in acute myeloid leukemia (AML) is critical to assess measurable residual disease (MRD). Next generation sequencing (NGS) assays are powerful tools for this, permitting interrogation of thousands of base positions across genomic regions of interest. However, PCR and sequencing errors in these assays limit accurate mutation detection at variant allele frequencies (VAF) below approximately 1%. Duplex sequencing (DS), an error-corrected NGS (ecNGS) method, greatly reduces such errors by relating original top and bottom DNA strands to make double stranded consensus sequences, thus enabling sensitive, specific, accurate, and precise detection of variants below 0.01% VAF.
Here, we report analytical validation of an updated DuplexSeq™ AML assay, a 36-gene panel informed by 2022 European LeukemiaNet (ELN) recommendations combined with kitted library preparation reagents and a cloud-based analysis pipeline. The assay uniformly targets regions that harbor AML-associated mutations: 82% of targeted regions exhibit sequencing depth >80% of the panel-wide mean and >99.5% of targeted regions exhibit depth >20% of the panel-wide mean.
These analytical studies also validate an improved “v2 chemistry” version of a DS library preparation kit. This improved chemistry provides an enzymatic fragmentation step that replaces time-consuming mechanical fragmentation methods while increasing the yield of duplex data for a given input mass of DNA sample and eliminating damage that results from mechanical fragmentation.
A contrived human genomic DNA sample carrying 26 variants targeted by the panel, DNA extracted from AML-positive peripheral blood (PB) and bone marrow aspirate (BMA) specimens, and DNA extracted from healthy normal PB specimens were used as test samples to execute limit of detection (LoD), limit of blank (LoB), accuracy, linearity, and repeatability & reproducibility studies. In this assay a duplex depth threshold of ≥20,000x is applied; ≥91.8% of the 42,605 genomic base positions targeted by the panel are consistently (in 95% of analyzed libraries) above this threshold.
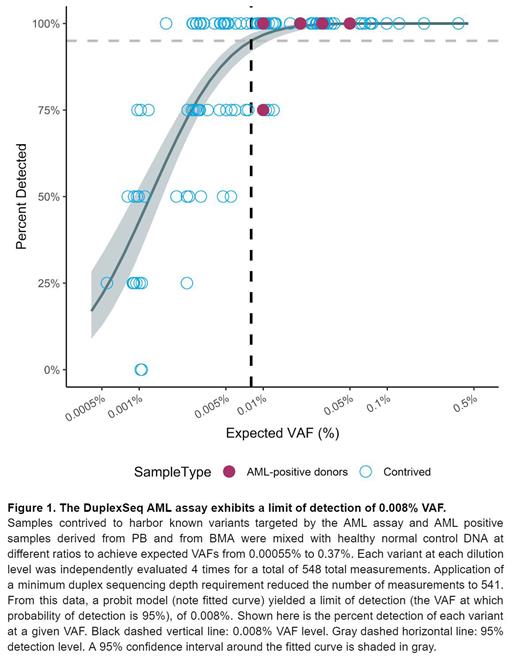
The LoD study assayed samples carrying variants from 0.37% to 0.00055% VAF. The assay demonstrated excellent sensitivity, with variants down to 0.00055% VAF detected. A probit model was used to estimate the VAF at which 95% of variants would be detected, establishing an LoD of 0.008% VAF for single-nucleotide variants (SNVs) and insertions and deletions (indels) (see Figure 1).
The LoB study assessed the background detection rate in DNA samples derived from healthy normal specimens. In >95% of sites surveyed, no variants were observed, establishing an assay LoB of 0 (0% VAF).
Accuracy of the assay was determined by interrogating PB and BMA samples from AML-positive and -negative donors along with contrived test samples. Results were compared to data acquired via orthogonal methods. For variants above the LoD of 0.008% VAF, accuracy was high, with overall percent agreement (OPA) of 98.3%, positive percent agreement (PPA) of 100.0%, and negative percent agreement (NPA) of 98.3%. Data were linear from 0.0016% VAF to 96.1% VAF with R 2 = 0.95. All 6 expected FLT3-ITD variants, and all 5 expected NPM1 insertions were detected by the assay.
Precision study data showed that the assay was highly repeatable, with OPA of 97.5%, PPA of 98.0%, and NPA of 97.3%. Reproducibility across 2 operators, 2 lots of reagents, and 3 independent runs of library preparation was also very high, with OPA of 97.5%, PPA of 98.8%, and NPA of 96.9%.
Current ELN guidelines provisionally define NGS-MRD positivity as ≥0.1% VAF, but recent evidence suggests lower frequency variants may be informative. With an LoD of 0.008% VAF for SNVs and indels, and an LoB of 0, this novel AML assay comprising an updated gene panel coupled with improved duplex sequencing chemistry and software represents a highly sensitive, specific, accurate, and precise ecNGS assay for detecting variants associated with AML MRD.
Disclosures
McElwain:Adaptive Biotechnologies: Ended employment in the past 24 months. Schmidt:Gilead: Other: spouse currently employed.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal